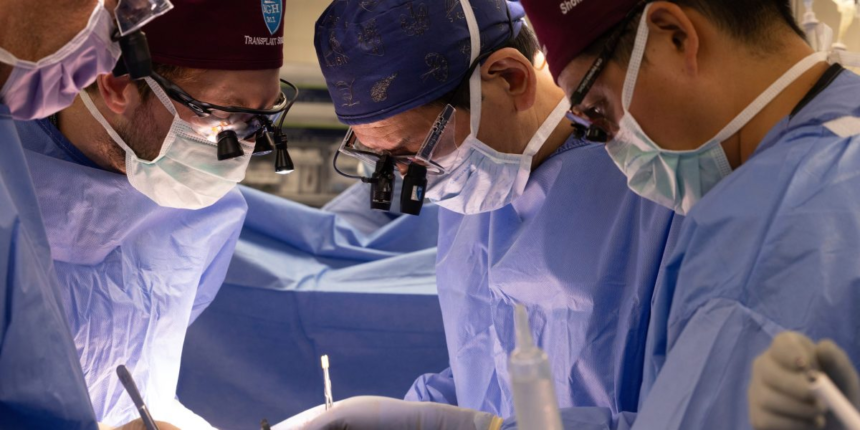
The 54-year-old New Hampshire man is faring well after his June 14 operation, doctors at Massachusetts General Hospital announced Monday.
“I really wanted to contribute to the science of it,” Bill Stewart, an athletic trainer from Dover, New Hampshire, told The Associated Press.
Based on lessons from the New Hampshire men and a handful of other one-off attempts, the Food and Drug Administration approved pig producer eGenesis to begin a rigorous study of kidney xenotransplants.
In New Hampshire, high blood pressure caused Stewart’s kidneys to fail but he had no other health problems. It can take up to seven years for people with his blood type to find a matching kidney from a deceased donor, and some would-be living donors didn’t qualify. After two years in dialysis, he heard about Mass General’s most recent xenotransplant recipient – Andrews – and applied to be the next candidate.
“I’ve always been a little bit of a science nerd,” Stewart said. Conscious of how new these experiments are, he sought out Andrews for advice and ultimately decided, “worst case scenario, they can always take it out.”
Thrilled to no longer have his time and energy sapped by dialysis, Stewart said he’s easing back into desk duties at work and visited his old dialysis clinic to “let everyone know I’m doing all right and maybe kind of give some people some hope.”
Riella, the kidney specialist, said Stewart had his anti-rejection drugs adjusted to counter an early concern and that Andrews has needed similar adjustments. He said it’s far too early to predict how long pig kidneys might be able to last — but it would be useful even if initially they can buy people time off dialysis until they get a matching human organ.
“A year, hopefully longer than that – that’s already a huge advantage,” he said.
The new eGenesis trial will provide gene-edited pig kidney transplants to 30 people age 50 or older who are on dialysis and the transplant list. Another developer of gene-edited pig organs, United Therapeutics, is about to start enrolling people in a similar FDA-approved study.